Regulatory Services
Regulatory services involves a range of activities aimed at ensuring that pharmaceutical products are produced, distributed, and marketed in compliance with regulatory standards, laws, and guidelines. These services are critical for safeguarding public health and ensuring the safety, efficacy, and quality of medications.
Here’s how regulatory services intersect with pharmaceutical manufacturing:

Compliance with Regulations
Bon-Heur Pharmaceuticals must adhere to a complex web of regulations set forth by regulatory agencies such as the Food and Drug Administration (FDA) in the United States, The European Medicines Agency (EMA) in Europe, and other similar authorities worldwide. Regulatory services help manufacturers interpret and implement these regulations effectively.

Product Registration and Approval
Before a pharmaceutical product can be marketed and sold, it must undergo rigorous review and approval by regulatory agencies. Regulatory services assist manufacturers in preparing and submitting applications for product registration, clinical trial approvals, and marketing authorizations. This process involves compiling comprehensive data on the safety, efficacy, and quality of the pharmaceutical product.

Quality Assurance and Good Manufacturing Practices (GMP)
Regulatory services ensure that pharmaceutical manufacturers adhere to Good Manufacturing Practices (GMP) and other quality assurance standards throughout the production process. This includes maintaining clean and controlled manufacturing facilities, validating production processes, conducting quality control testing, and implementing robust documentation and record-keeping procedures.
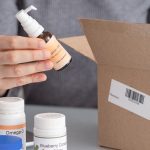
Labelling and Packaging Compliance
Pharmaceutical products must have accurate and informative labelling and packaging that comply with regulatory requirements. Regulatory services help manufacturers develop labelling and packaging materials that provide essential information to healthcare professionals and patients while meeting regulatory standards for safety, efficacy, and product identification.

Post-Market Surveillance and Reporting
Regulatory services extend beyond the initial approval process to encompass post-market surveillance and reporting of adverse events, product defects, and quality issues. Pharmaceutical manufacturers are required to monitor the safety and performance of their products in the marketplace and report any adverse events to regulatory authorities in a timely manner.

Regulatory Audits and Inspections
Regulatory agencies conduct routine inspections and audits of pharmaceutical manufacturing facilities to assess compliance with regulatory requirements and quality standards. Regulatory services assist manufacturers in preparing for and responding to regulatory inspections, addressing deficiencies, and implementing corrective actions to ensure ongoing compliance.

Supply Chain Management
Pharmaceutical manufacturers must ensure the integrity and traceability of their supply chains to prevent counterfeit products, contamination, and other risks. Regulatory services help manufacturers establish and maintain robust supply chain management practices, including supplier qualification, raw material sourcing, and distribution controls.

Regulatory Intelligence and Strategy
Regulatory services provide manufacturers with insights into evolving regulatory requirements, industry trends, and best practices. This includes monitoring changes in regulations, guidance documents, and enforcement priorities to inform regulatory strategies and ensure proactive compliance with emerging requirements.
We are providing regulatory services involve navigating a complex regulatory landscape to ensure that our products meet the highest standards of safety, efficacy, and quality throughout the entire product lifecycle, from development and manufacturing to distribution and post-market surveillance.
Contact Us
- Mfg. Unit : Plot No. 131, Sector- 6A, IIE-SIDCUL, Haridwar-249 403, Uttarakhand, India.
- Mfg. Unit : Plot No. 130B, Sector- 6A, IIE-SIDCUL, Haridwar-249 403, Uttarakhand, India.
- Mumbai Office : 18, 3rd Floor, Shreenath Building, Kalbadevi, Mumbai - 400 002
- Office: +91 - 9520865925
- Email: bdm.bonheurpharma@gmail.com
- https://bonheurpharma.in/

